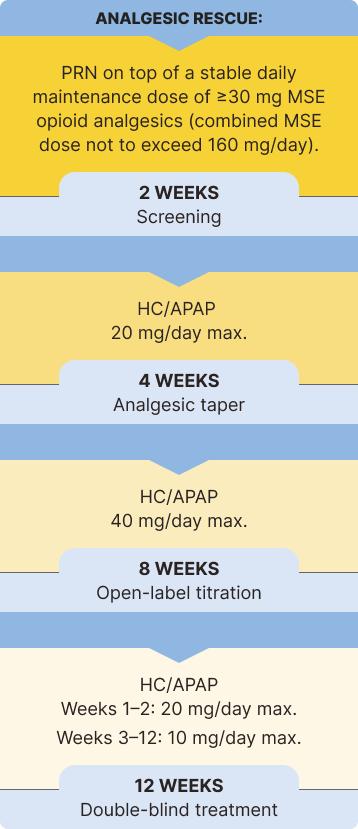
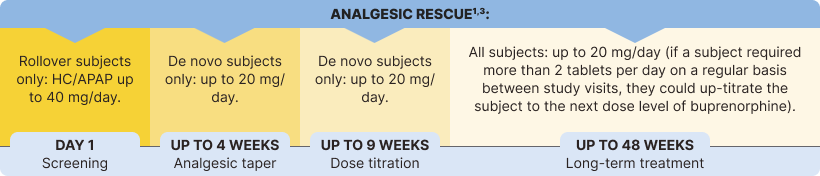
The safety and efficacy of BELBUCA in opioid-experienced patients with moderate to severe chronic pain were evaluated in a multicenter, double-blind, placebo-controlled, enriched-enrollment, randomized-withdrawal study3*
Study Objective: To determine the analgesic efficacy of BELBUCA every 12 hours (Q12h) in opioid-experienced patients (including those taking up to 160 mg/d MSE) with moderate to severe chronic low back pain using around-the-clock opioid analgesics for an extended period1,3
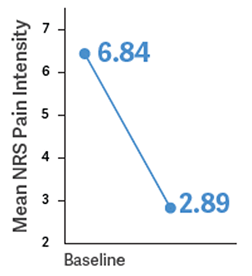
Primary Endpoint: Determine the change in mean average NRS daily pain intensity score from baseline to week 12 of the double-blind treatment period1,3
BELBUCA has also been studied in opioid-naive patients. Only doses up to 450 mcg q12h were studied in opioid-naive patients.1
No head-to-head trial vs CII opioids was conducted.
NRS=numerical rating scale, where 0=no pain and 10=worst pain imaginable.
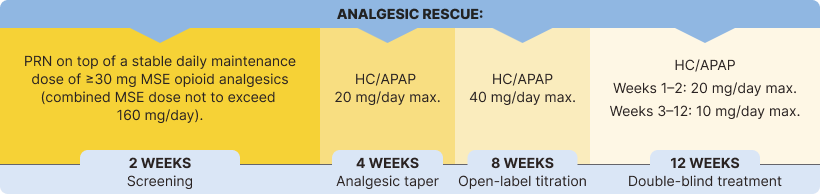
The percentage of patients using rescue medication at week 12 was significantly lower in the BELBUCA group than the placebo group in the double-blind treatment period3
Immediate-release opioids were used as rescue medication throughout the BELBUCA study for breakthrough pain3,7
No head-to-head trial vs CII opioids was conducted.
Respiratory depression can occur at any time during opioid therapy, especially when initiating and following dosage increases with BELBUCA. Consider this risk when selecting an initial dose and when making dose adjustments.
HC/APAP=hydrocodone/acetaminophen; MSE=morphine sulfate equivalent; PRN=as needed.